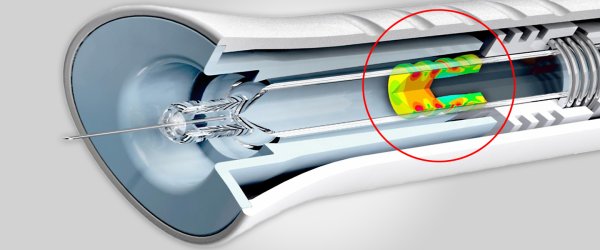
Stress Engineering Services is an industry leader in failure analysis for medical, consumer and pharmaceutical devices, as well as a variety of other sectors. We employ a systematic approach to the identification, evaluation, and mitigation of failures in mechanical, electrical, and electromechanical devices. Our approach to failure identification complements current FMEA (Failure Modes and Effects Analysis) techniques employed by device manufacturers/OEMs. We accomplish this by applying deliberate, physics-based quantitative methods towards the assessment of device and system behavior under conditions that may lead to the failure of key end user functions.

Our analytical and testing experts are skilled in a variety of disciplines and fields including material engineering, mechanical engineering, electrical engineering, biomedical engineering, polymers, and packaging. This collective force of professionals and experience, along with our comprehensive testing capabilities, enables us to provide complete and timely results that can help prevent future failures.