Understanding the Reynst Combustion Pot: The Simplest Pulse Jet Engine

by: Larry Matta, Ph.D., P.E., C.F.E.I.
The “jelly jar combustor” is a simple version of a Reynst combustion pot. It is a basic pulse jet engine that operates without moving parts. Though inefficient and loud, it provides an excellent demonstration of combustion and fluid mechanics principles.
What is a Reynst Combustion Pot?
Named after Dutch engineer Willem Reynst, this combustor is a pulsed combustion chamber that exhausts the combustion products and brings in a fresh charge of air through a single hole. Some of Reynst’s designs were more complicated, incorporating a tuned exhaust pipe among other things. The jelly jar version is as simple as it gets, consisting of:
- A glass jar with a hole in the lid
- A small amount of liquid fuel (e.g., isopropanol)
- A self-sustaining combustion cycle
This setup results in repeated cycles of combustion, exhaust, and fresh air intake, similar to pulse jet engines used in early aviation.
How It Works
This simple pulse combustor follows a three-step breathing cycle:
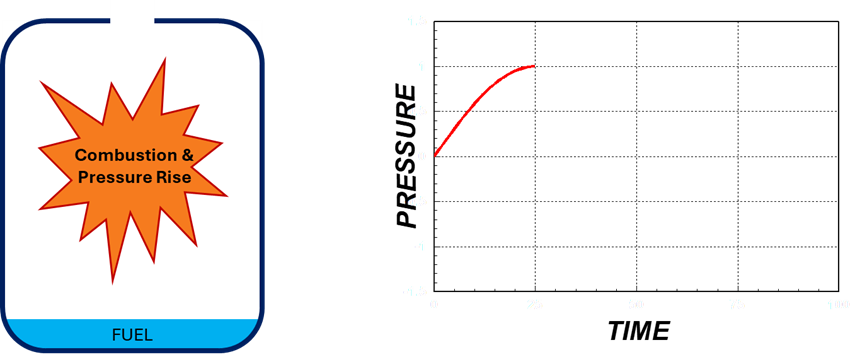
1. Rapid Combustion and Pressure Rise
- Rapid burning of fuel vapor and air, causing a sudden increase in pressure.
- Hot pressurized gases exit through the hole, starting the exhaust phase.
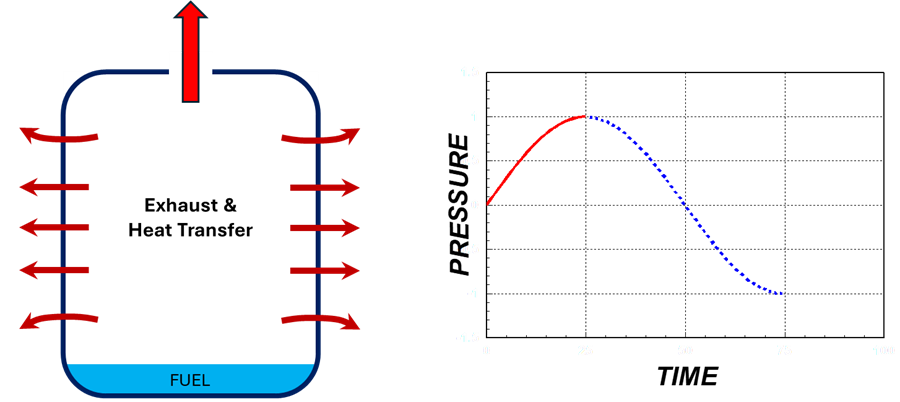
2. Exhaust and Heat Transfer
- Hot gases heat the jar and evaporate more fuel.
- As the combustion product gases leave, pressure inside the jar drops.
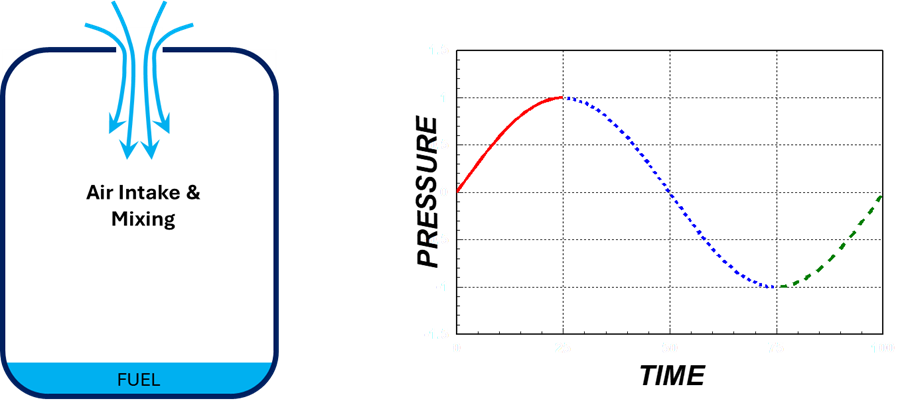
3. Air Intake and Mixing
- Fresh air rushes in, mixing with fuel vapors.
- The mixture reignites from residual heat and flame, restarting the cycle.
This pulsating process continues until the fuel is depleted or the jar cracks from thermal stress.
The Science Behind It
Though mechanically simple, the combustor involves complex fluid dynamics and combustion physics:
- Flame Propagation: Ignition generates a pressure wave that expels gases.
- Turbulent Mixing: Incoming air promotes thorough fuel-air mixing.
- Heat Transfer: Heating the jar and the fuel cools the gas while accelerating fuel evaporation.
- Timing and Resonance: Proper synchronization is crucial for sustaining combustion.
Applications and Limitations
Attempts to use larger Reynst combustors in water heaters and furnaces failed due to extreme noise levels and thermal stress.
Limitations:
- Inefficiency: Not enough pressure to create a strong jet for propulsion.
- Thermal Stress: Glass jars may crack from uneven heating. Good for watching though!
- Excessive Noise: Larger versions generate intense sound waves and significant vibrations.
Why Study the Reynst Combustion Pot?
Despite its limitations, the jelly jar combustor is a valuable educational tool for combustion science. It showcases how a self-sustaining combustion cycle occurs without mechanical components.
However, safety precautions are essential when literally playing with fire. The jar gets extremely hot, and improper handling of flammable fuels can be dangerous. Always use safety glasses, a fire extinguisher, and a controlled testing area.
Conclusion
The Reynst combustion pot is a simple yet intriguing example of a pulsed combustion system. While impractical for real-world applications, it provides insight into fluid dynamics, air-fuel mixing, and resonance.
As combustion research advances, revisiting experimental systems like the jelly jar combustor helps engineers better understand energy conversion and engine design.
Rapid Combustion & Pressure Rise
Exhaust & Heat Transfer
Air Intake & Mixing

Lawrence M. Matta, PhD, PE, CFEI – Staff Consultant, Houston Office
Dr. Matta, is a mechanical engineer specializing in fluid and solid mechanics, thermodynamics, and combustion. With extensive experience in pipeline stress analysis, transient flow problems, and forensic investigations of fires, explosions, and equipment failures, he brings deep technical expertise to engineering challenges. He is a Staff Consultant at Stress Engineering Services and a certified fire and explosion investigator.




Leave a Comment
You must Register or Login to post a comment.